– PARADIGM ™ program comprises two 12-week randomized, placebo-controlled studies (APPROACH and EMBRACE) and a long-term extension study (EXTEND) –
– APPROACH ™ : A P hase III, P lacebo-Controlled, R andomized, Double-Blind Trial of O ral Doses of CYB003 to A ssess C ombined Safety and Efficacy in H umans with Major Depressive Disorder has been initiated and will enroll 220 patients at 36 clinical sites across the U.S. and Europe; topline results expected in 2026 –
– EMBRACE ™ : An E fficacy and Safety, Phase III, M ulti-center, Double- B lind, R andomized Controlled Study Comparing 2 A ctive and 1 Inactive Oral Doses of C YB003 in E ligible Participants with Major Depressive Disorder is expected to begin in 1H25 –
– EXTEND: a Phase III Open Label Exten sion Study with Optional Additional Doses of CYB003 to Assess the Safety and Long-term Efficacy in Participants With Major D epressive Disorder is expected to begin 12 weeks after commencement of APPROACH and EMBRACE, respectively –
-12-month efficacy data from Phase 2 CYB003 MDD study expected in Q4 2024 –
– CYB003 in development for the treatment of MDD has a total addressable market of >300 million people worldwide 1 and 21 million in the U.S. 2 –
– Cash totaled C$154.3 million as of September 30, 2024 –
This news release constitutes a “designated news release” for the purposes of Cybin’s prospectus supplements each dated August 23, 2023, to its short form base shelf prospectus dated August 17, 2023, as amended December 22, 2023 and April 8, 2024.
TORONTO–(BUSINESS WIRE)– Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“ Cybin ” or the “ Company ”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the initiation of PARADIGM TM , its Phase 3 pivotal program evaluating the efficacy and safety of CYB003 for the adjunctive treatment of Major Depressive Disorder (“ MDD ”). The program name, PARADIGM, represents the Company’s belief that CYB003 could have the potential for a paradigm shift in the treatment of depression. The Company also today reported unaudited financial results for its second quarter ended September 30, 2024.
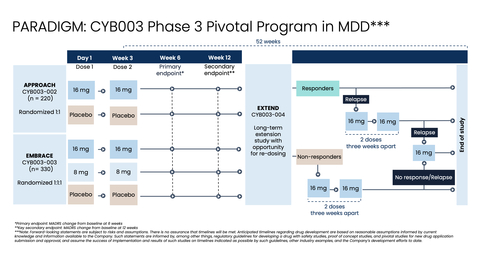 Phase 3 PARADIGM Pivotal Program Design (Graphic: Business Wire)
Phase 3 PARADIGM Pivotal Program Design (Graphic: Business Wire)
“Just three years after filing an Investigational New Drug application for CYB003, the initiation of our Phase 3 program is a truly significant and gratifying milestone,” said Doug Drysdale, Chief Executive Officer of Cybin. “Following a highly collaborative and thorough design and review process with the U.S. Food and Drug Administration, we believe that PARADIGM incorporates appropriate protocols that proactively address some of the challenges encountered by peers developing molecules with similar mechanisms of action by (i) recruiting from the larger MDD population; (ii) administering CYB003 as an adjunctive treatment and not requiring patients to titrate off their existing antidepressants; and (iii) utilizing a 12-week blinded period to maximize the number of patients that remain in the study through the blinded stage. Our innovative approach represents a potential ‘paradigm’ shift, moving away from the daily treatment of depression symptoms and toward an intermittent, more durable treatment like CYB003 that could potentially change the course of the disease. Our clinical team has accomplished an extraordinary amount in a short time, and we are eager to continue investigating CYB003’s potential to provide long-lasting relief from depressive symptoms and disrupt the standard of care in MDD,” concluded Drysdale.
About the Phase 3 PARADIGM Pivotal Program
The Company’s Phase 3 program comprises three pivotal efficacy studies:
Pivotal study 1 (APPROACH):
- Participants (n=220) will be randomized 1:1 to receive either 16 mg of CYB003 (n=110) or inactive placebo (n=110). Each study arm will evaluate a two-dose regimen, with doses administered three weeks apart. The study will enroll patients suffering from moderate to severe MDD (MADRS≥24) who are on a stable dose of antidepressant medication but are responding inadequately.
- The primary endpoint will be change in depressive symptoms as measured by change in MADRS from baseline at six weeks after the first dose.
APPROACH will enroll participants at 36 clinical sites across the U.S. and Europe.
Pivotal study 2 (EMBRACE):
- Participants (n=330) will be randomized 1:1:1 to receive 16 mg of CYB003 (n=110), 8 mg of CYB003 (n=110), or inactive placebo (n=110). Each arm will evaluate a two-dose regimen, with doses administered three weeks apart. The study will enroll patients suffering from moderate to severe MDD (MADRS≥24) who are on a stable dose of antidepressant medication but are responding inadequately.
- The primary endpoint will be change in depressive symptoms as measured by change in MADRS from baseline at six weeks after the first dose.
EMBRACE is expected to enroll at 48 clinical sites, with minimal site overlap with the APPROACH study.
Pivotal study 3 (EXTEND):
- Participants from APPROACH and EMBRACE will roll over into EXTEND (up to n=550) after the completion of the 12-week, double-blind, placebo-controlled treatment periods. During EXTEND, all participants who did not respond to treatment in the APPROACH and EMBRACE studies or who relapse during the EXTEND study will be eligible to receive an additional two doses of CYB003 (16 mg) administered three weeks apart. Participants who do not respond to these two doses or relapse again will be eligible to receive an additional single 16 mg dose of CYB003.
Across all three studies, raters will be remote, independent, and blinded with no information on the dose received or the participant’s dosing experience. Effects during the dosing session will be firewalled to ensure that the study team stays blinded.
“Our unique Phase 3 pivotal program design has been informed by the impressive Phase 2 (four-month) data showing rapid, robust improvements in symptoms of depression with a single dose of CYB003, and durable effects four months after two doses with a 75% remission rate in the 16mg dose group. For our pivotal program, we have preserved the two-dose regimen used in our Phase 2 study, given the strong durability results seen to date,” said Amir Inamdar, Chief Medical Officer of Cybin. “The need for improved treatments for mental health disorders has never been greater. We believe that our Phase 3 program can build on the positive results demonstrated in Phase 2 to-date and could potentially lead to the approval and commercialization of a novel treatment modality whose effects are consistent and durable for patients with MDD.”
Positive Phase 2 Four-Month Efficacy Data for CYB003 in MDD
- Robust and sustained improvements in symptoms of depression with two doses of 12 mg or 16 mg of CYB003:
- Mean reduction from baseline in the MADRS total score was approximately 22 points from baseline in both dosing cohorts.
- Approximately 75% of the patients were responders (>/= 50% improvement in MADRS scores) following two doses of 16mg.
- 75% of patients on 16 mg were in remission from depression following 2 doses (MADRS score </= 10).
Safety and tolerability:
- CYB003 was well tolerated with no drug-related serious adverse events.
- All adverse events were mild or moderate in intensity.
- No incidents of suicidal ideation or behavior.
- No discontinuations due to adverse events.
Upcoming Clinical Milestones and Future Studies Across Cybin’s Pipeline:
CYB003 – Deuterated Psilocin Program 3
- 12-month efficacy data from Phase 2 MDD study expected Q4 2024.
- The first pivotal study APPROACH has been initiated, with topline results expected in 2026.
- The second pivotal study EMBRACE is expected to begin in the first half of 2025.
CYB004 – Deuterated DMT Program 3
- Dosing is underway in a Phase 2 study in generalized anxiety disorder with topline safety and efficacy results expected in Q1 2025. CYB004 is being developed as a novel intramuscular formulation expected to deliver an experience lasting approximately 90 minutes.
Second Quarter Fiscal Year 2025 Financial Information
- Cash totaled C$154.3 million as of September 30, 2024.
- With the completion of offerings and a combination of the Company’s current cash position, and assuming the exercise in full of warrants issued pursuant to certain of the Company’s offerings, the Company has access to over C$217.7 million.
- Cash-based operating expenses consisting of research, general and administrative costs totaled C$24.8 million for the quarter ended September 30, 2024, compared to C$12.4 million in the same period last year.
- Net loss was C$57.2 million for the quarter ended September 30, 2024, which includes a non-recurring, non-cash component related to share-based compensation, compared to a net loss of C$11.9 million in the same period last year.
- Cash flows used in operating activities were C$25.9 million for the quarter ended September 30, 2024, compared to C$11.5 million in the same period last year.
About Cybin
Cybin is a late-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options to address the large unmet need for people who suffer from mental health conditions.
With industry leading proof-of-concept data, Cybin is working to change the mental health treatment landscape through the introduction of intermittent treatments that provide long lasting results. The Company is currently developing CYB003, a proprietary deuterated psilocin program, in Phase 3 development for the adjunctive treatment of MDD and CYB004, a proprietary deuterated DMT program in a Phase 2 study for generalized anxiety disorder. The Company also has a research pipeline of investigational, 5-HT-receptor focused compounds.
Founded in 2019, Cybin is operational in Canada, the United States, the United Kingdom, the Netherlands and Ireland. For company updates and to learn more about Cybin, visit www.cybin.com or follow the team on X, LinkedIn, YouTube and Instagram.
Notes:
- World Health Organization. (2017). Depression and other common mental disorders: global health estimates. World Health Organization. https://iris.who.int/handle/10665/254610.
- https://www.nimh.nih.gov/health/statistics/major-depression
- There is no assurance that timelines will be met. Anticipated timelines regarding the initiation, advancement and results of clinical trials are based on reasonable assumptions informed by current knowledge and information available to the Company.
Cautionary Notes and Forward-Looking Statements
Certain statements in this news release relating to the Company are forward-looking statements and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe” or “continue”, or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding the Company’s plan to enroll participants in its Phase 3 trials of CYB003; release topline results from the APPROACH study in 2026 ; initiate the EMBRACE study in the first half of calendar 2025; initiate the EXTEND study 12 weeks following commencement of the APPROACH and EMBRACE studies, respectively; release of 12-month efficacy data from Phase 2 CYB003 study in Q4 2024; release of Phase 2 topline data for CYB004 in the first quarter of calendar 2025; enroll 48 clinical sites for the EMBRACE study; the exercise in full of warrants issued pursuant to certain of the Company’s offerings; and the Company’s proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health disorders.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the spread of a pandemic on the Company’s operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in each of the Company’s management’s discussion and analysis for the three and six month periods ended September 30, 2024 and the Company’s annual information form for the year ended March 31, 2024, which are available under the Company’s profile on www.sedarplus.ca and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Although the forward-looking statements contained in this news release are based upon what management of the Company believes, or believed at the time, to be reasonable assumptions, the Company cannot assure shareholders that actual results will be consistent with such forward-looking statements, as there may be other factors that cause results not to be as anticipated, estimated or intended. Readers should not place undue reliance on the forward-looking statements and information contained in this news release. The Company assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change, except as required by law.
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The U.S. Food and Drug Administration, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Rigorous scientific research and clinical trials are needed. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin’s performance and operations.
Neither the Cboe Canada nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
Investor & Media Contact:
Gabriel Fahel
Chief Legal Officer
Cybin Inc.
1-866-292-4601
irteam@cybin.com – or – media@cybin.com
Source: Cybin Inc.












